Robotic, Laparoscopic / Minimally Invasive Ventral, Incisional and Umbilical Hernias (For Visceral Contact)
A next generation lighter weight barrier mesh formed from non-woven microfibers of polypropylene partially impregnated with non-porous silicone to produce a strongly integrated abdominal mesh that minimizes visceral tissue contact long term.

- Superior Tissue Incorporation
- Low profile mesh for ease of introduction and placement
- Flexible memory mesh aiding in placement and fixation
- Fully trimmable, customizable mesh
- More patient comfortable mesh long term avoiding stiff abdomen syndrome
- Strongly incorporated mesh which is a permanent tissue attachment barrier in just 12 days
- Mesh with serous drainage holes to assist with seroma prevention
- Minimal shrinkage mesh
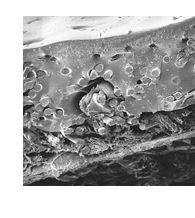
See applicable SURGIMESH® IFU for information on Precautions and Possible Complications.
Key Configurations
Circular + Suture
Tintra C-10/12/15 – Umbilical/Ventral

Rectangle
Tintra R-1415 – Small Umbilical/Ventral
Ellipse
Tintra E-1522 – Incisional/Ventral
Oval
Tintra O-2636 – Large Abdominal Wall
A multitude of other configurations of SURGIMESH® XB are available.
See our SURGIMESH® XB Inventory Guide or check with your local BG Medical Representative.
Support Information
- SURGIMESH® XB Brochure
- SURGIMESH® XB Testimonials
- SURGIMESH® XB Ramshaw CQI Study AHS 2016 Meeting Abstract
- SURGIMESH® XB YUNIS 1st WORLD CONF. HERNIA SURGERY ABSTRACT
- SURGIMESH® XB YUNIS ACOS ABSTRACT
- SURGIMESH® XB 75 Day Re-look Case Report
- SURGIMESH® XB 180 Day Re-look Case Report
- SURGIMESH® Influence of Mesh Structure on Surgical Healing in Abdominal Wall Hernia Repair – World Biomaterials Congress
- SURGIMESH® XB Product Summary
- SURGIMESH® XB IFU

